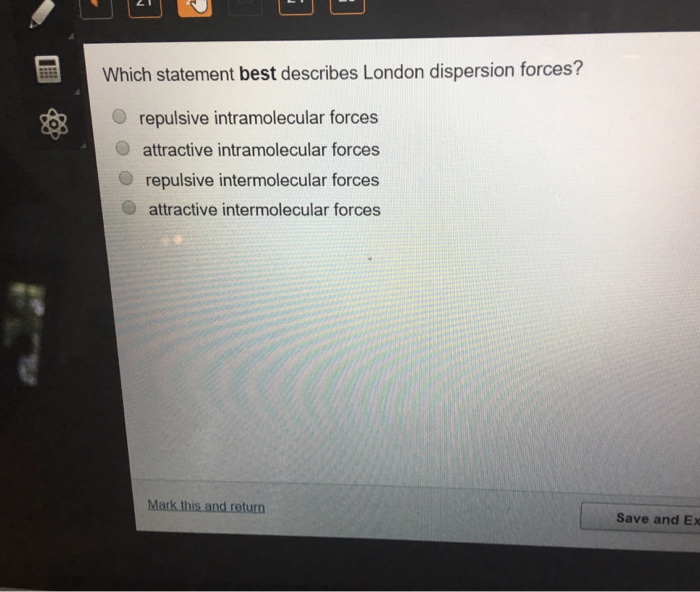
Which Statement Best Describes London Dispersion Forces
Forces within covalent molecules that hold them together. Repulsive intermolecular forces D.
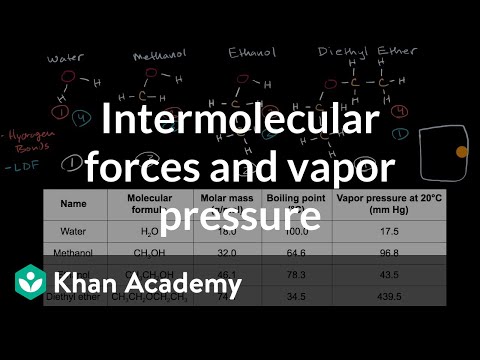
Intermolecular Forces And Vapor Pressure Video Khan Academy
Attractive intermolecular forces 2.

. As the number of electrons in a molecule increases the london dispersion force increases. A binary compound is found to be non-polar. Covalent bonds within a network solid.
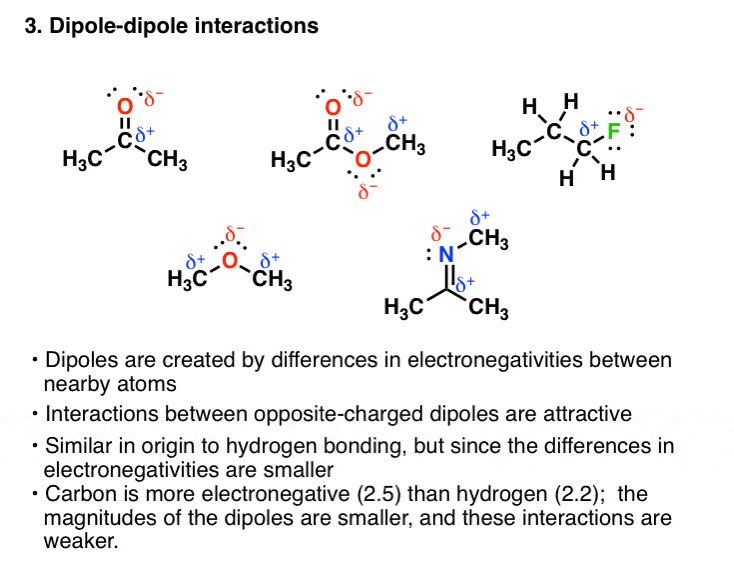
The valence electrons are easily delocalized. The hydrogen-bonding forces in NH3are stronger than those in H 2 O. The dipoles are permanent separations of charges.
According to this C option is correct. We review their content and use your feedback to keep the quality high. This force is the weakest among all the intermolecular forces.
Which statement about london dispersion forces is true. The force arisen from induced dipole and the interaction is weaker than the dipole-dipole interaction. Molecules that have only London dispersion forms will always be gases at room temperature 25 C.
The dipoles are instantaneous and temporary. Forces are a relatively new discovery which leads to them being misunderstood C. These forces are weaker than intermolecular forces.
Attractive intramolecular forces C. London dispersion forces are stronger between molecules of 1-butanol than between molecules of 2-methyl-2-propanol. Repulsive intramolecular forces O attractive intramolecular forces repulsive intermolecular forces.
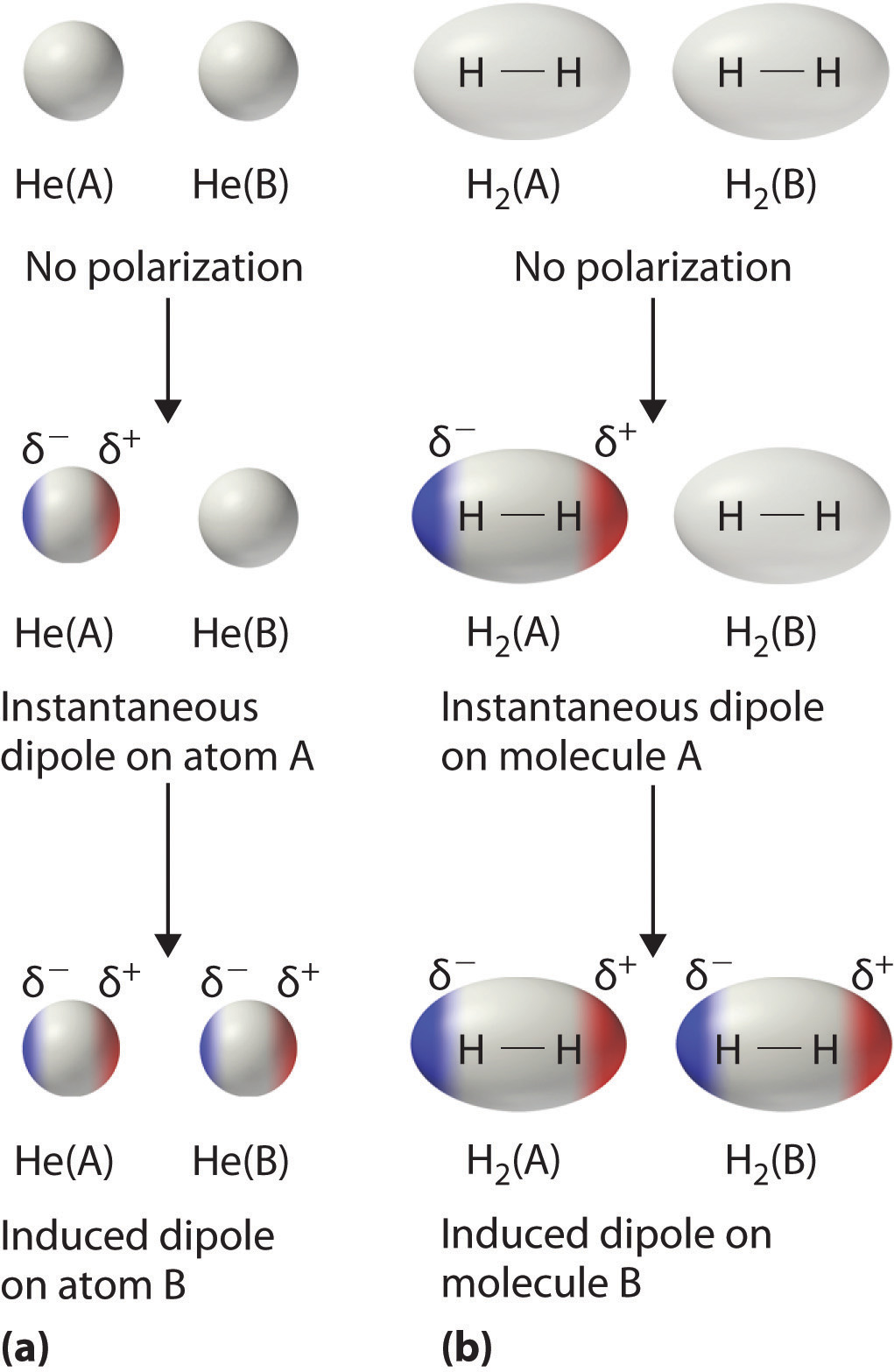
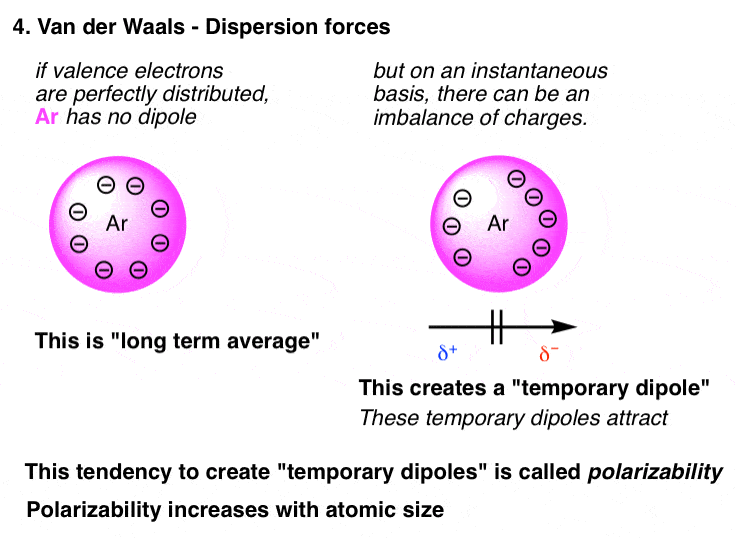
London Dispersion Forces Definition. The forces of attraction between molecules which hold them together are called the intermolecular force of attraction. London dispersion forces are the only type of intermolecular force that nonpnlar molecules exhibit.
Which statement best describes london dispersion forces. 3 Show answers Another question on Chemistry. 1 point the sphere that.
These forces always operate in any substance. Bekas 84K 1 year ago 7 0 It is also called as van der Waals force. Which statement best describes the dipoles that cause London dispersion forces.
Temporary dipole-induced dipole interactions Londondispersion forces C. Features of dispersion force. The following compounds have similar molar masses.
Repulsive intramolecular forces B. Repulsive intramolecular forces B. Which of the following statements about intermolecular forces is are true.
Which statement best describes the effect of low ionization energies and low electronegativities on metallic. London dispersion forces are. It is a temporary force that happens when electrons of two adjacent atoms occupy positions that make atoms form dipoles which are temporary dipoles.
Which statement best describes London dispersion forces. Electrostatic forces between ions. Carbon and hydrogen have very similar electro negativity values so we typically think of the arrangement of a hydrocarbon thes both being hydrocarbons as being non polar and the inter molecular forces that predominate our London dispersion forces.
The forces of dispersion may be repellent or attractive. Which statement best describes ionic bonding. 18 5 2 taskmasters London dispersion forces is the weakest intermolecular force.
Two forces are the only forces acting on a 78 kg object which moves with an accelera- tion of 45 ms2 in the positive y direction. The dipoles contain a hydrogen atom. The key characteristics of the dispersion force London dispersion force Long-range dispersion forces can be efficient from wide distances 10nm all the way down to interatomic distances.
Forces are weaker because they energy is dispersed among a greater number of electrons B. Get 1-on-1 help from an expert tutor now. Chemistry 18092019 0730 Joshuafranklindude.
The first set of two that is provided is propane and butane. Summarize possible ways in which phases of matter could combine to. Alexandria3498 SHOW ANSWER London dispersion forces is the weakest intermolecular force.
Attractive i naomibush naomibush 04102017 Chemistry High School answered Which statement best describes London dispersion forces. It is the electrostatic attraction between positive ions and delocalized. Which statement best describes London dispersion forces.
Forces do not have the electron proton interaction of a. Up to 24 cash back B. This is also referred as dipole-dipole attraction.
The london dispersion force decreases. Which statement best describes London dispersion forces. Which statement best describes london dispersion forces.
Hydrogen Bonding London Dispersion Forces Dipole-Dipole 24 Which statement best describes why forces are weaker than bonds. Attractive forces between separate molecules. One of the forces acts in the positive x direc- tion and has a magnitude of 14 N.
Bonds between hydrogen and oxygen atoms in water molecules. The dipoles are caused by a difference in electronegativity. Repulsive intramolecular forces attractive intramolecular forces repulsive intermolecular forces attractive intermolecular forces.
Which of the following statements best describes the geosphere. The force of attraction which is a result of shifting of electrons in two adjacent atoms even in molecules that are non polar and create the atoms to temporary dipoles is termed as London dispersion force. So we can say that covalent bond ionic bond coordination bond are the intra-molecular force of attraction which form within a molecule.
What is the order of increasing boiling points. The dispersion force does not obey a simple power law.
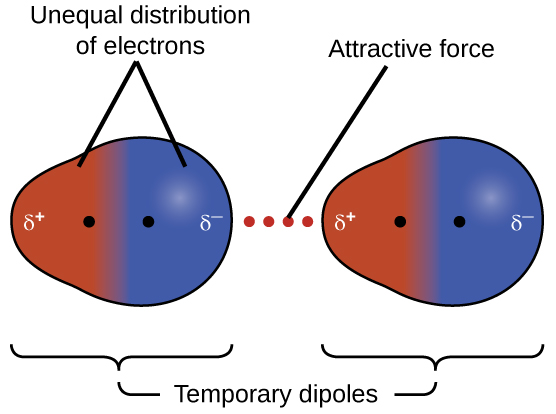
10 1 Intermolecular Forces Chemistry

London Dispersion Force Chemistry Lessons Intermolecular Force Ap Chemistry
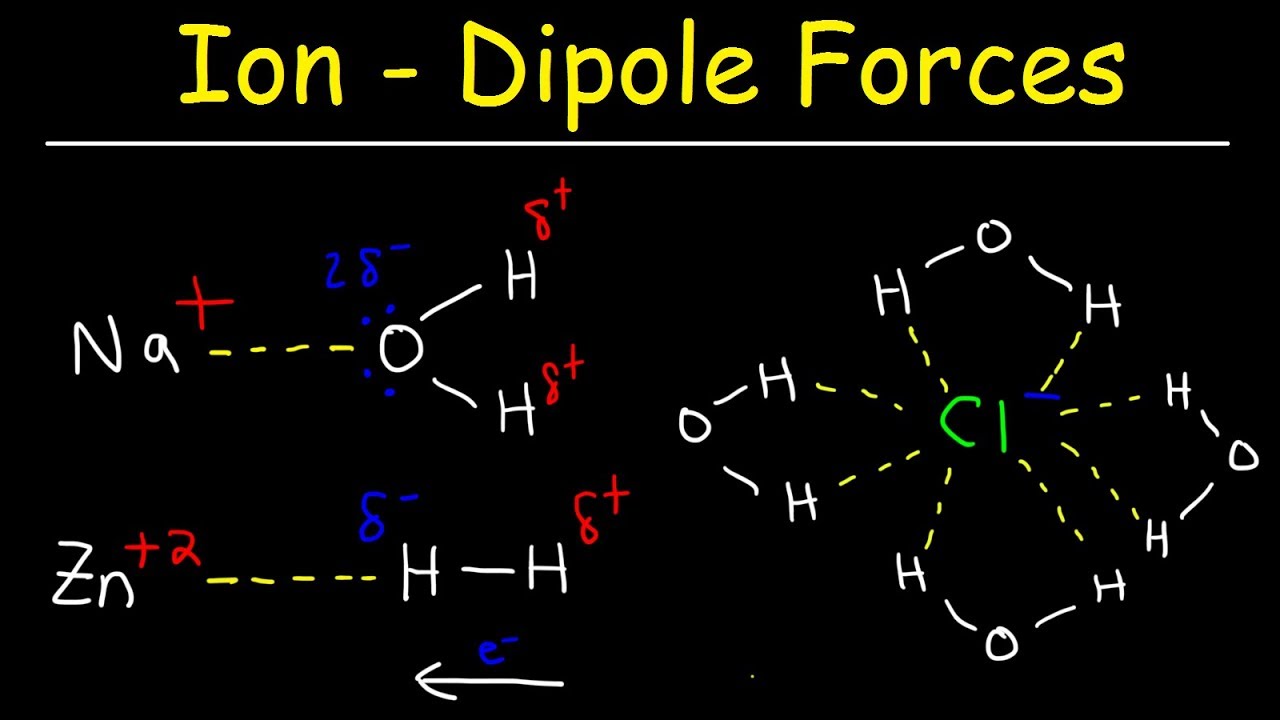
London Dispersion Forces Temporary Dipole Induced Dipole Interactions Intermolecular Forces Youtube
Could Someone Explain London Dispersion Forces Quora
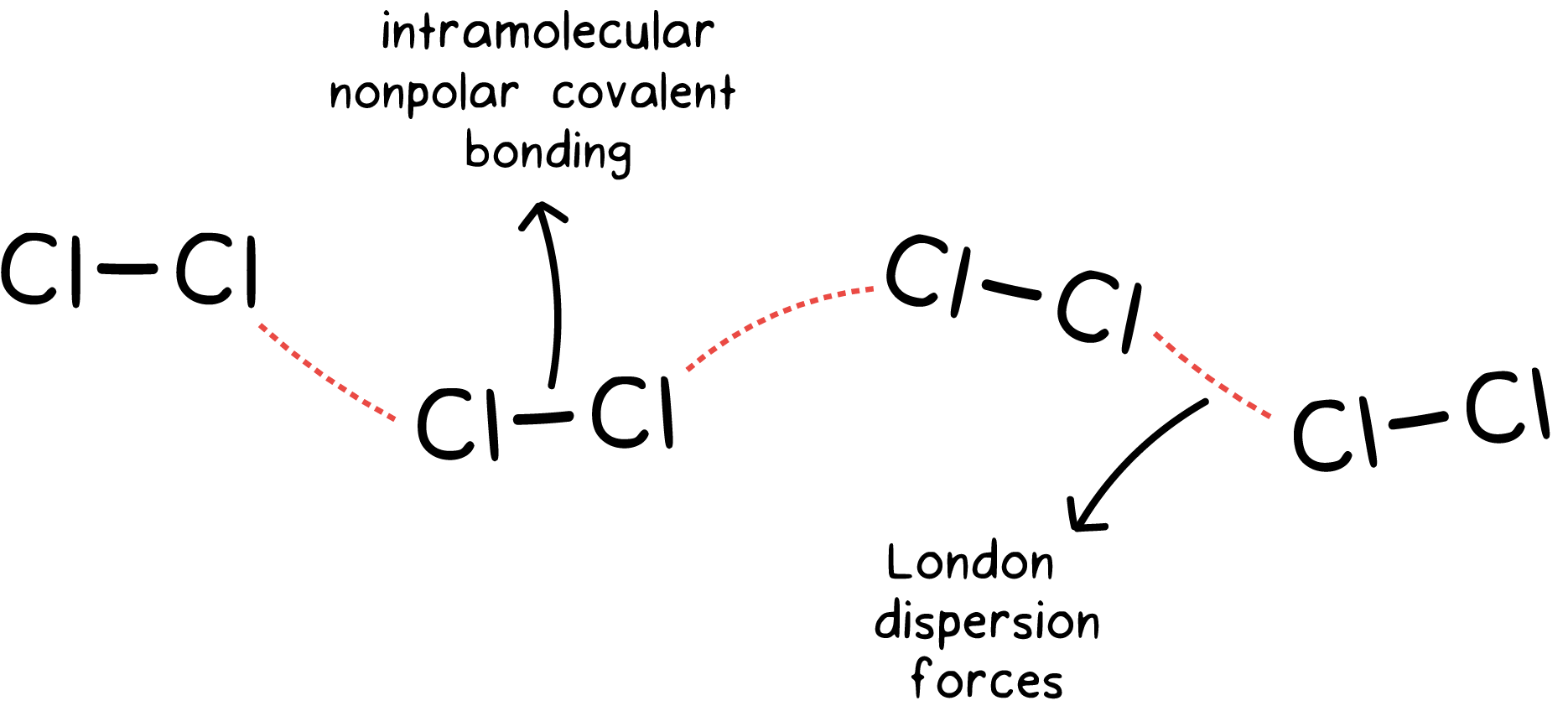
Intramolecular And Intermolecular Forces Article Khan Academy
Solved Which Statement Best Describes London Dispersion Chegg Com

Solved Which Of The Following Statements Best Describes The Chegg Com
11 3 Intermolecular Forces The Forces That Hold Condensed Phases Together Chemistry Libretexts

London Dispersion Forces Youtube

London Dispersion Forces Temporary Dipole Induced Dipole Interactions Intermolecular Forces Youtube
The Four Intermolecular Forces And How They Affect Boiling Points

11 3 Intermolecular Forces The Forces That Hold Condensed Phases Together Chemistry Libretexts

Van Der Waals Forces Chemistry For Non Majors
The Four Intermolecular Forces And How They Affect Boiling Points

Dipole Dipole Forces Video Khan Academy
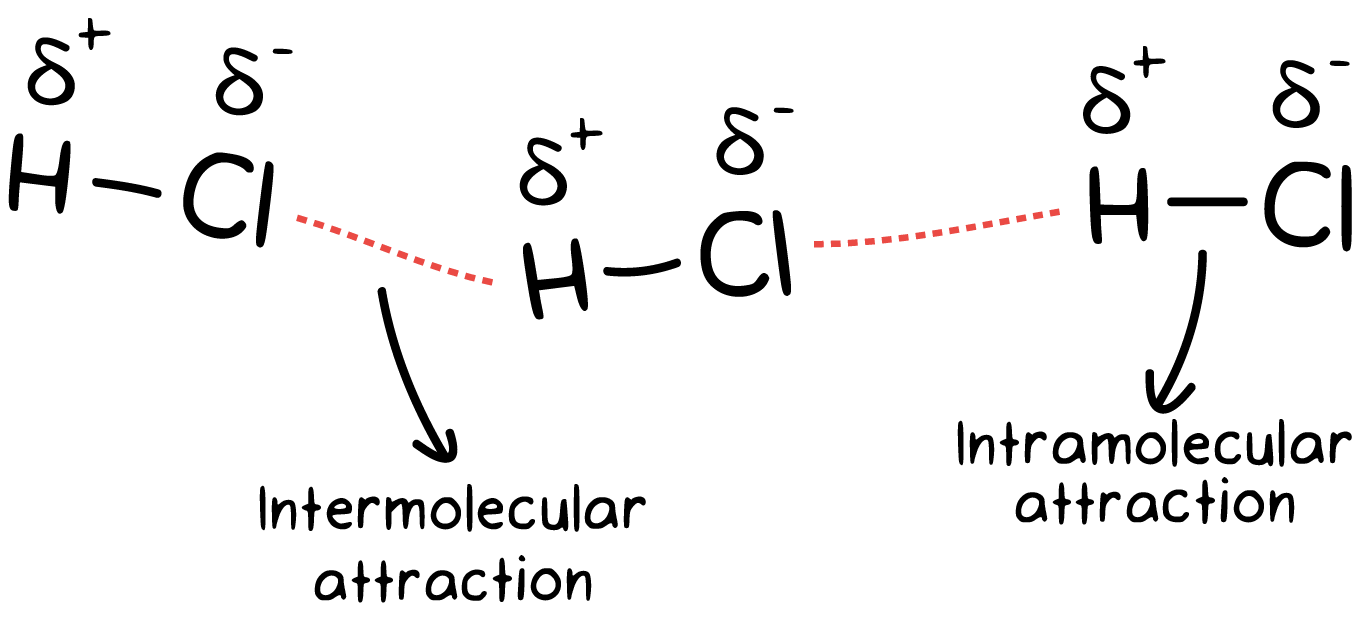
Intramolecular And Intermolecular Forces Article Khan Academy
How Do London Dispersion Forces Form Between Polar Molecules Quora

London Dispersion Forces Types Of Intermolecular Forces Vander Waal S Force Of Attraction Chimica
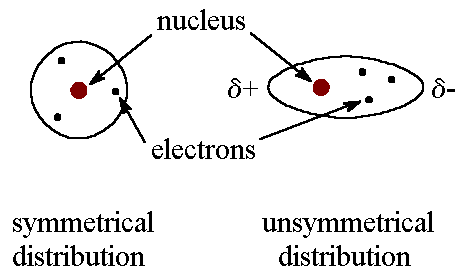
Comments
Post a Comment